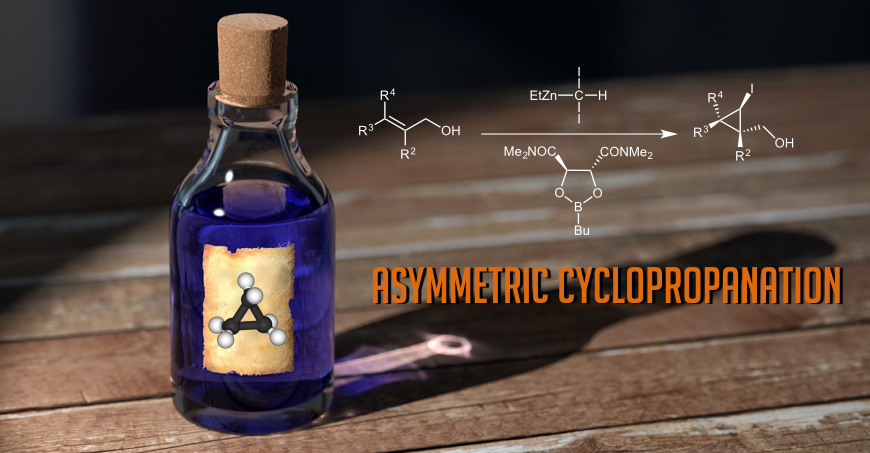
For more than 2 decades, our research group has made seminal contributions and has been internationally recognized in the area of the enantioselective cyclopropanation of alkenes and the preparation of enantiopure cyclopropanes. Among others, we have found highly useful methods and new reagents leading to cyclopropanes with better chemoselectivities, higher enantiocontrol and milder reaction conditions. Since 2010, we have published more than 20 articles as well as several other book chapters and we have been invited to give numerous talks at various symposia, universities and companies worldwide. In the literature, the dioxaborolane mediated cyclopropanation reaction of allylic alcohols using zinc carbenoids is often referred to as the “Charette asymmetric cyclopropanation reaction.” Our methods have been featured as key steps in numerous total syntheses and are widely used by the scientific community (academia and industry). Our work has even been highlighted in C&E News, Angew. Chem., Chemtracts and in Synfacts.
We have shown that amides can be used as precursors to a variety of other functional groups (thioamides, thiazolines, amidines, esters, etc.) as well as novel heterocycles. The key feature is a highly chemoselective activation with triflic anhydride. We have developed highly chemoselective reductions of secondary and tertiary amides under mild conditions, a work which has been selected as Synfacts of the Month and listed in the 50 most-read articles in Nature Chem. in early 2012. More recently, this strategy has been successfully used to generate nitrogen-containing heterocycles (Org. Lett. 2015, 17, 3386) and 3-aminoimidazo[1,2-a]pyridines.
We have described several efficient methodologies that can be efficiently used to make hetereocycles (Acc. Chem. Res. 2013, 46, 412) and substituted cyclopropane derivatives via novel C–H functionalization reactions. Given the importance of the cyclopropane ring in medicinal chemistry, we expect that the impact will be significant since our strategy allows for easy diversification.
Current methods for peptide synthesis and other difficult amide bond formation, although general and high yielding, have inherent limits, including concerns about waste, expense, and the epimerization of stereocenters. Most amide and peptide coupling reactions are still carried out using a stoichiometric excess of expensive coupling reagents that can also be potentially hazardous. We described an alternative and unprecedented approach to peptide synthesis involving a chemically ligatation of both amino acid subunits through a temporary silicon tether. This work has already caught the attention of the community and will be widely used to generate amide.

Many reagents for the synthesis of complex structures are toxic, unstable or not heasily handled. The same is true with some reactive intermediates. Flow chemistry techniques make it possible to avoid these issues, because the hazardous intermediate can be used as it is produced, avoiding accumulation and handling. Over the years, we have reported several methods that produce very reactive intermediates that can be used in situ to generate important molecular scaffolds. Among those, we reported an hydroxylation of ary iodides promoted by copper tubing (Synlett 2014, 1409), a difluorocyclopropanation process (Org. Lett. 2016, 18, 1988) and the preparation of aryldiazomethane reagents (Angew. Chem., Int. Ed. 2017, 56, 837). The latter method is a metal-free continuous flow process that can be used for the production of aryldiazomethane solutions in a non-coordinating solvent from easily-prepared, bench-stable sulfonylhydrazones. We are also developing new API manufacturing processes using flow chemistry.